Is CBD a food supplement, a medicine, or an aroma product?
If you’re reading this article, it’s most likely because you’re worried about the recent changes to the labelling of cannabidiol (CBD) products. Maybe you’ve been using CBD in oil or capsules but are now asking yourself ‘is it still safe to ingest them?’. Or you’ve heard of CBD products and want to try them (especially following the preliminary reports that CBD can help fight off Covid infection) but are confused as to why some are labelled ‘aroma product, for external use only’.
We’re here to help. At the Cannabis College, we are dedicated to offering transparency and accurate information about all matters pertaining to cannabis – CBD labelling included. Here we break down the story of how cannabidiol has been classified, and why some companies have changed the descriptions of their CBD products.


The story of CBD classification
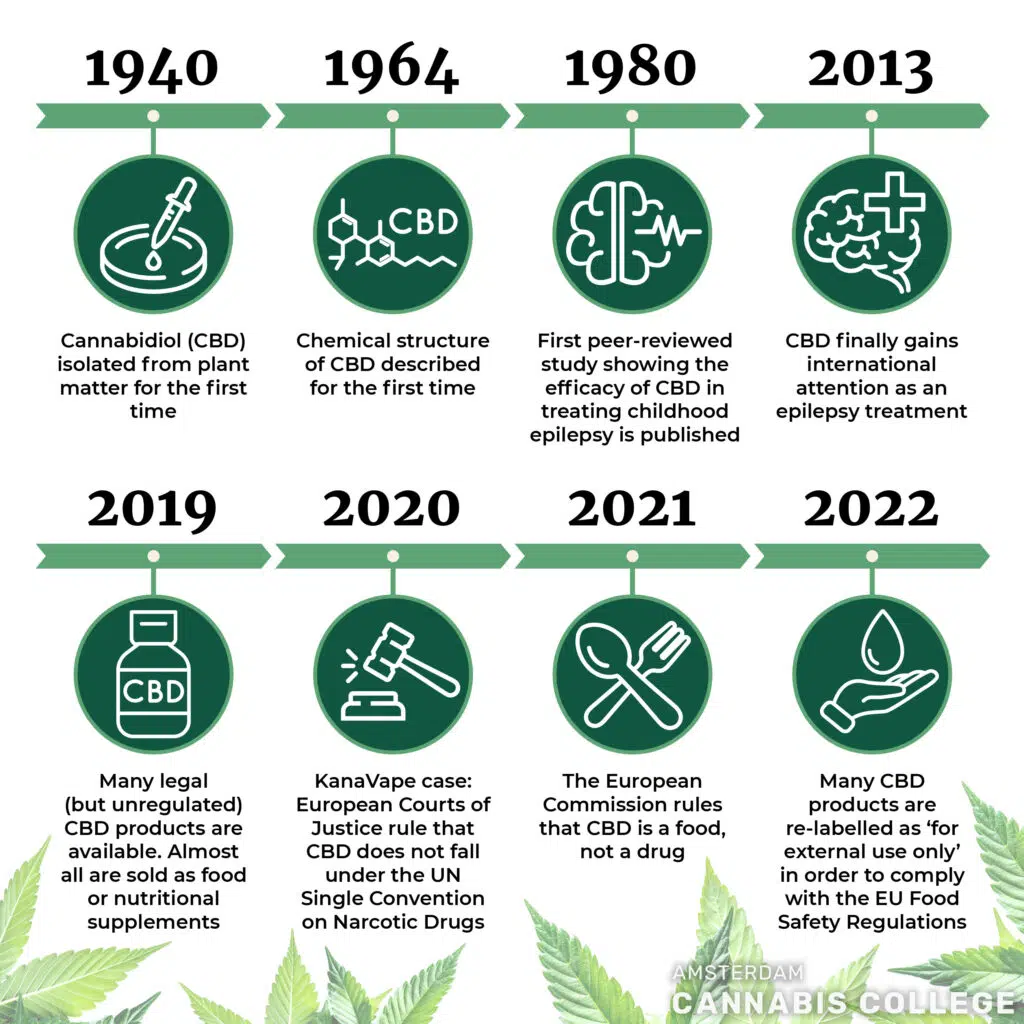
In the beginning, CBD was simply a cannabinoid. First discovered in 1940, it was initially thought to be a precursor of THC but we now know that they are ‘sibling’ cannabinoids, both produced from cannabigerolic acid (CBGA). As CBD had no psychotropic, mood-altering or mind-expanding effects, THC (which does) took the research spotlight for many years.
The first peer-reviewed scientific evidence for CBD as an effective treatment for childhood epilepsy was published in 1980 but, amazingly, went mostly unremarked. The stigma around cannabis was still too great for it to be widely reported on.
It wasn’t until the early 2010s, following the legalisation of medicinal cannabis in various US states, that the medicinal qualities of CBD began to draw attention. The remarkable success it had on alleviating the symptoms of epilepsy, especially in children suffering from the previously untreatable Dravet Syndrome, sparked a wave of worldwide interest and research. Many countries revised their laws to allow the manufacture and use of CBD medications.
Industrial hemp is naturally high in CBD
High amounts of CBD are naturally present in industrial hemp. This meant CBD could be produced without having to grow or process strains of cannabis that are illegal due to their THC content. Industrial hemp cannot have more than a tiny amount of THC. The exact percentage varies by country but all are less than 0.05%.
As a substance cannot legally be called a medicine unless it has passed many stringent tests, the new products made with CBD from industrial hemp could not be sold as medicines, nor as having any medicinal value. It is (rightly!) illegal to make medicinal claims for something unless there is proper data to back up those claims.
Equally, only licensed pharmacies can dispense medicines. Any company selling CBD products as medicines would need to have a pharmacy license, which virtually none of them did. These rules are the reason for the rise in ‘wellness’ products – ‘wellness’ is such a vague term that virtually any product can legally claim to enhance it.
It’s important to note right from the beginning that in practically all cases, the formulation of the products has remained the same. Only the labelling is different.
There are no legal quality controls, no governing bodies […] to protect the consumer from inadequate or misleading products.

Medicinal claims cannot be made for CBD products unless they are licensed medicines
The simple solution was to sell CBD products without making any medicinal claims for them (although some companies did so anyway). The ‘new’ cannabinoid was added to everything from lip balm to sportswear. Legislation swiftly followed to set limits on the amount of THC allowed in such products. This was simple, as THC was already a controlled substance.
However, there are no limits or requirements on the amount of CBD that products have to contain. There is no minimum threshold of CBD that a product must meet in order to call itself a ‘CBD product’. Lawyer, legal counsel, and cannabis law expert Bob Vink describes CBD as ‘the new gold in cannabis country’ – many people are rushing to make money from it, but so far there are no legal quality controls, no governing bodies, and very little to protect the consumer from inadequate or misleading products.
The KanaVape case – a pivotal moment for CBD legislation
In Europe, this situation has led to attempts to control CBD using existing laws. The most important case in terms of new legislation was that of a French vape and e-cigarette company called Kanavape. Kanavape were selling vaporiser ‘pens’ containing CBD liquid. The French public prosecutor attempted to stop them, using the terms of the UN Single Convention on Narcotic Drugs. KanaVape appealed the case, and in 2021 they won.
The European Court of Justice ruled in favour of KanaVape on the basis of the following arguments:
● CBD is a cannabis product, but it is not psychoactive in the way that THC is
● The Single Convention on Narcotic Drugs was created to legislate narcotic and psychoactive drugs
● Therefore CBD does not fall under the intention of the narcotic laws
● Buying and selling CBD products is perfectly legal in many countries in Europe
● Therefore prosecuting companies that produce and sell CBD products in France is against the European laws covering the free trade of goods.
This ruling of the European Court is why even more CBD products have flooded onto the market in many European countries over the past couple of years. At the same time, the governments of these countries continue to look for ways of regulating this new range of products.
Prosecuting companies that produce and sell CBD products in France is against the European laws covering the free trade of goods.

Cannabidiol can be qualified as food, provided EU Food Safety Regulation are also met.
The European Commission rules CBD is not a narcotic drug
The Kanavape case is also significant because it changed the European Commission’s categorisation of CBD. In reaction to the European Court’s ruling in the Kanavape case, the Commission reversed its previous preliminary decision that plant-derived CBD should be considered narcotics in the EU and stated:
“The European Commission has noted that cannabidiol should not be considered as a drug within the meaning of the United Nations Single Convention on Narcotic Drugs of 1961 and that cannabidiol can be qualified as food, provided that the other conditions of the EU Food Safety Regulation are also met.”
This decision basically took CBD out of the jurisdiction of the European Courts of Justice and handed it to the EU Commission on Food Safety to deal with. It was their job to decide on the status of CBD as a food supplement or nutritional product. Somewhat illogically, they have decided that it’s a ‘novel food’.
European Commission on Food Safety rules CBD is a ‘novel food’
According to the European Commission on Food Safety, CBD and foods that it is added to (for example, hempseed oil) are classed as ‘novel foods’. Novel foods are defined as ‘food that had not been consumed to a significant degree by humans in the EU before 15 May 1997’.
Obviously, humans have been consuming CBD (along with THC) in any food and drink containing cannabis leaves or flowers, or their trichomes, for a long time. Cannabis brownies are mentioned in an American cookbook from 1954. The first printed cookbook, De Honesta Voluptate Et Valetudine (Of Honest Voluptuousness and Health) published in Italy in 1474, contains a recipe for ‘a health drink of cannabis nectar’. The Indian drink bhang dates back to around 1000 BCE.
Unfortunately, the legal status of these foodstuffs means that they were not ‘officially’ available or consumed in Europe before 1997, and so they are not recognised by the European Commission. We do not, as the kids say, have the receipts. The conditions of the EU Food Safety Regulation mentioned earlier cannot be met until CBD as a food product is tested and authorised.
CBD can still be sold in non-food products, for now
However, if the products it appears in are not food, then there is no objection to their sale from the EU Commission on Food Safety.
This is the latest hoop that CBD products have to jump through: they cannot be sold as medicinal, and now they cannot be sold as nutritional either. This is the reason for the recent relabelling of CBD products as ‘for external use only’, despite the product remaining the same.
Even if evidence was found that CBD was consumed to a significant degree in Europe prior to 1997, there is a further complication. In 2020 the entry for Cannabis sativa L. in the EU Food Commission lists was changed. Since then, only seed-derived products are considered food. The leaves and flowers are left in a ‘grey zone’.
This effectively removes CBD from the jurisdiction of the Food Commission, without making it clear who (if anyone) now takes the responsibility for it. The fate of CBD products in Europe remains unsure.
CBD products […] cannot be sold as medicinal, and now they cannot be sold as nutritional either. This is the reason for the recent relabelling of CBD products as ‘for external use only’.

It is unlikely that CBD will ‘go underground’
However, there are various reasons to expect CBD products to remain on the market. Firstly, they are immensely lucrative for business. According to the group Prohibition Partners, ‘The [global] Over-The-Counter CBD market is expected to be worth US $5.8 billion [€5 billion] in 2020 with the potential to grow by 16% over the review period to US $6.79 billion [€5.85 billion].’
Because the CBD market is so massive there is a lot of incentive to find a solution to its legal status and regulations. It is unlikely that this market will ‘go underground’, especially as CBD does not have the mind-expanding properties of THC and therefore does not elicit the same fearful reaction from some groups of people.
On the contrary, there are millions of people using and benefitting from CBD products who may never have used or come into contact with cannabis in any other form. They may even still oppose the recreational and spiritual use of it. But they are very unwilling to give up the supplements that have apparently alleviated their stress, insomnia, arthritis, inflammation, menstrual and menopausal issues, and general aches and pains.
For now, it seems likely that CBD products will continue to be sold as being ‘for external use only’ in places where that is required by law.
Frequently asked questions
Is it hard to grow Cannabis?
Cannabis is an easy plant to grow. It is not called a weed for no reason. Refer to our Basic growing guide for more information.
What Cannabis seeds should I choose?
This is a difficult question to answer. Seeds come in different forms: regular seeds producing both male and female plats, feminised seeds, and autoflowering seeds. Each has its advantages and disadvantages. Choosing the ones best suited to you depends on what your are looking for from your cannabis plant and your level of experience.
How long does it take to grow Cannabis?
This depends on the Cannabis variety you are growing and its environment. Life cycles can vary from two and a half months for autoflowering varieties to more than 18 months for certain sativa varieties.